683
Views & Citations10
Likes & Shares
Immunotherapy based on targeting the monoclonal antigen-4 associated with cytotoxic T lymphocytes (CTLA4), programmed cell death protein 1 (PD-1) and its ligand (PDL-1) are recognized as the standard treatment for an increasing number of indications, and with its frequent use, side effects become ever more present in the daily clinical practice. Examples of such therapies are Ipilimumab and Nivolumab, either as a single agent or combined. The use of these medications may result in immune-mediated adverse reactions, sometimes serious and fatal, which reinforces the imperative need for early diagnosis and correct management of immune-related toxicities (IOT). We report a rare and severe case of non-bacterial auto-immune cystitis after Ipilimumab-nivolumab for metastatic renal cell carcinoma. The severity of symptoms and its importance in clinical management are highlighted and should be promptly recognized.
Keywords: Immunotherapy, Renal cell carcinoma, Polaciuria, Immune-related toxicities, Sedimentoscopy
INTRODUCTION
Immunotherapy based on targeting the monoclonal antigen-4 associated with cytotoxic T lymphocytes (CTLA4), programmed cell death protein 1 (PD-1) and its ligand (PDL-1) are recognized as the standard treatment for an increasing number of indications, such as melanoma, kidney cancer, MSI-high tumors, among others. With its frequent use, IOT become ever more present in the daily clinical practice, while some forms of IOT are still not well characterized [1].
Ipilimumab, a monoclonal antibody, acts by binding to CTLA4 that is expressed on the surface of activated CD4 and CD8 T lymphocytes, resulting in inhibition of the interaction between CTLA4 and the CD80/ CD86 target. This increases the immune response of T cells, including the activation and proliferation of these cells [2].
This medication can trigger immune-mediated adverse reactions, which in some cases can be serious and fatal. These may involve any organ; however, the most serious and common adverse reactions are: enterocolitis, hepatitis, dermatitis, endocrine disorders and neurotoxicity. Usually, the majority of adverse events occur in the beginning of treatment [3].
Nivolumab is a human IgG4 anti-PD1 monoclonal antibody that acts by binding to PD-1 expressed in T-cells and inhibits the binding between PDL-1 and 2 expressed in tumor cells to the PD-1 receptor, thereby enhancing the immune response through further expressed and activated T-cells [2]. This agent is generally associated with a lower degree of toxicity when compared to Ipilimumab [1].The combination of Ipilimumab and Nivolumab (Ipi/nivo), although more active in some tumor types is associated with a higher incidence of autoimmune effects, leading to severe toxicity and treatment discontinuation in a great number of patients [4]. We report and unusual and severe case of non-bacterial auto-immune related cystitis after Ipi/nivo for metastatic renal cell carcinoma, and similarly to other 3 cases reported in literature, this event occurred during or immediately after immune-mediated colitis. This is the first case reported after ipi/nivo, as the others occurred after Nivo alone. The severity of symptoms and its importance in clinical management should be promptly recognized by prescribing physicians.
CASE REPORT
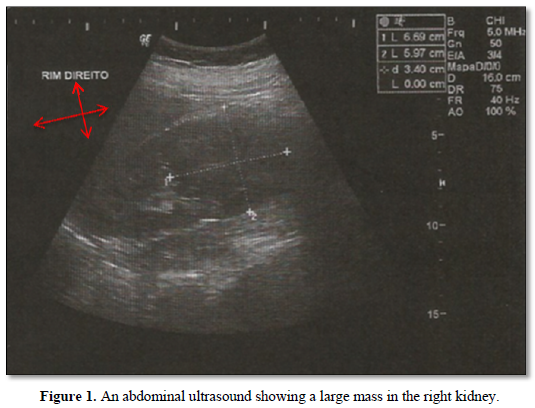
A.P.G, female, 38 years old, business owner, with no family history of cancer, with no comorbidities and asymptomatic. In March 2018, during routine checkups, an abdominal ultrasound showed a large mass in the right kidney (Figure 1). She underwent a laparoscopic radical right nephrectomy in June 2018. The final pathology report revealed renal cell carcinoma, clear cell subtype, Fuhrman 2, stage pT2 pNx. The patient recovered well from surgery, ECOG performance status at the time was 0.
During follow-up, in September 2018, a supraclavicular lymph node was palpable in physical exam, investigation with Positron Emission Tomography (PET-CT) revealed extensive lymphatic spread, with uptake in several lymphatic chains: left supraclavicular, mediastinal, para-aortic, para-tracheal, pre-carinal, sub-carinal, aortopulmonary window and right hilar. These findings were interpreted as inoperable disease recurrence; therefore, the patient was started on first line treatment for metastatic disease with Nivolumab (3 mg/kg) combined with Ipilimumab (1 mg/kg) intravenously every 3 weeks for 4 cycles, followed by Nivolumab (3 mg/kg) intravenously every 2 weeks until disease progression or prohibitive toxicity [5].
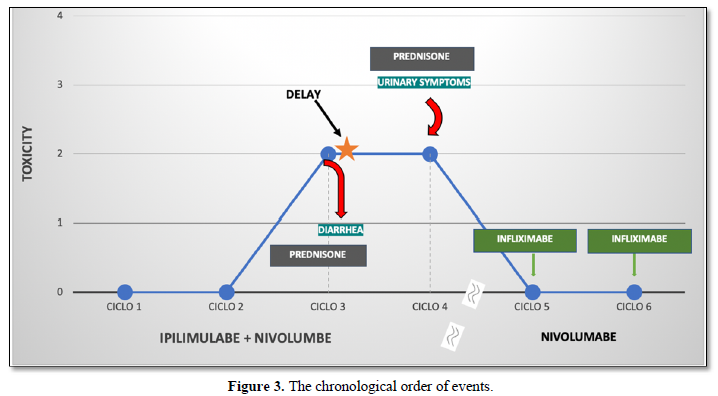
Treatment started on November 2018, and was well tolerated up into the third cycle, when the patient presented with daily multiple episodes of liquid diarrhea, associated with abdominal pain, which was diagnosed as immune mediated colitis grade 1-2. Initially, she was given 60 mg of oral prednisone daily and immunotherapy was postponed until full recovery. Despite corticosteroid use after several weeks, she persisted with symptoms, so Infliximab was associated to manage the colitis.
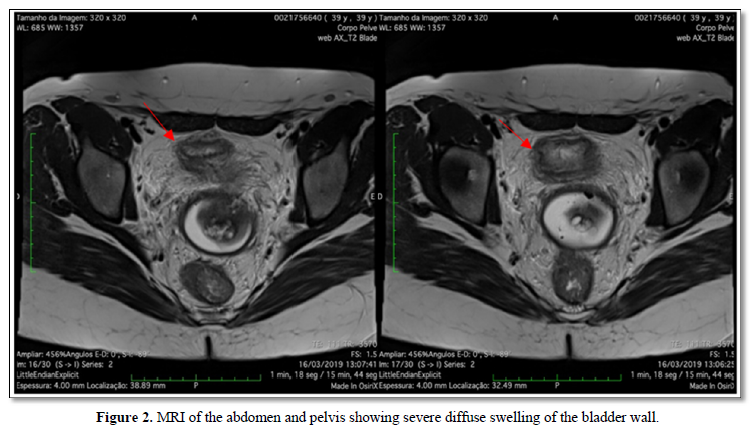
After complete remission of symptoms with Infliximab, the patient received the fourth and final dose of Ipi/nivo combination, but suddenly developed intense irritative urinary symptoms such as dysuria, strangury, vesical tenesmus, low abdominal pain that wouldn’t cease with proper antibiotic therapy nor with analgesics. Urinary sedimentoscopy showed massive pyuria and automated cultures for common germs tested negative multiple times. A Magnetic Resonance of the abdomen and pelvis was performed, which only showed diffuse thickening of the bladder wall (Figure 2). Oral prednisone 1mg/kg daily was started and symptoms gradually decreased in the following days, but did not stop leaving the patient with chronic pain. She remained on Nivolumab 3mg/kg intravenously every 2 weeks, butInfliximab was added with complete
resolution of cystitis symptoms and improve in several urine tests showing decrease in pyuria. Until now having received 4 cycles without further toxicities or new symptoms, except for mild diarrhea (grade 1). In Figure 3, we can observe the chronological events.
DISCUSSION
Immunotherapy based on targeting the monoclonal antigen-4 associated with cytotoxic T lymphocytes (CTLA4), programmed cell death protein 1 (PD-1) and its ligand (PDL-1) are recognized as the standard treatment for an increasing number of indications [1]. First-line therapy of advanced and metastatic renal cell carcinoma (mRCC) was modified in 2017 after the Phase III CheckMate 214 trial showing benefit for ipi/nivo in treatment-naïve patients with intermediate or high-risk mRCC. The group treated with the combination of ipi/nivohad a high rate of objective response, greater overall survival as well as longer lasting responses when compared to patients on standard therapy with Sunitinib, these results were consistent regardless PDL-1 expression [6].
In contrast with the superior results with the combination of ipi/nivo, the strategy to activate the immune system in order to combat neoplastic cells can also activate lymphocytes against healthy tissues, which constitutes the rationale for immune mediated toxicities. Concerning all-grade adverse events reported in the Checkmate 214 trial, 93% of the patients in the combination arm experienced any event(of those, 79,9% were typical immune-mediated events), while in the Sunitinib arm, that number was 97%. When considering only the most severe events (grades 3,4), these were less common in the experimental arm, although this group also experienced a higher percentage of discontinuation due to toxicities (22% vs. 12%). Most of the discontinuation occurred in the first 4 cycles of therapy (induction phase) [6].
Immune related adverse events may affect virtually any tissue in the human body, patients should be aware of any sign or symptom and be routinely monitored by the prescribing physician and assessed with laboratory or other exams. When symptoms do develop, infections or possible disease progression should be excluded, and it is recommended to initiate steroid use as soon as possible and delay or interrupt immunotherapy as needed by the severity and outcome of the event. Long term tumor response was observed even in patients requiring temporary or permanent ceasing of treatment [5,7,8].
Adverse events associated with Ipilimumab commonly reported in literature are usually graded 1-2 in severity and occur mainly in the first 8-12 weeks of treatment, with cutaneous toxicity being the earliest [9], while it is common that Nivolumab (and other anti-PD 1 drugs) relates to fatigue [1,10], rash, diarrhea, pruritus, hyporexia and nausea [10]. When these agents are combined, immune related toxicity may occur within the first 4 months of treatment [11], mainly: cutaneous involvement (pruritus, dermatitis), endocrine (thyroiditis), gastrointestinal (colitis), hepatic, renal and pulmonary. A special concern to severity must be noted in this setting-it is reported that immune-related events of any grade occur up to 95% of patients, whereas grade 3 or higher, 55% [12].
The literature is very limited when it comes to rare toxicities such asmyocarditis, encephalitis and many other sites were reported as immune-relates.
Non-bacterial cystitis after the use of Nivolumab was previously reported in 2 case reports, including a total of 3 patients.
In the first case, a 50-year-old male with stage-IV lung cancer presented with polaciuria, dysuria and diarrhea after 7 cycles of Nivolumab. Urinary sedimentoscopy revealed massive pyuria and serial urine cultures were negative. Symptoms resolved after the use of systemic corticosteroids, with recurrence after new exposure to the drug in the 8th cycle. Treatment was discontinued due to disease progression, with complete regression of symptoms [4].
In the second case, a 62-year-old man with stage IV squamous cell carcinoma developed dysuria, polaciuria and macroscopic hematuria after the third cycle of Nivolumab. Cystoscopy showed diffuse enanthem and erosion of the bladder mucosa, biopsy revealed that the mucosal epithelium had been completely discarded, and edematous interstitial changes were observed. Grade 3 dysuria and hematuria were attributed to Nivolumab after the exclusion of other causes. The patient was re-exposed to the drug 40 days after initially scheduled and treatment was continued with concomitant administration of systemic corticosteroids until discontinuation in cycle 8 due to disease progression [6].
Finally, a 60-year-old man with stage IV lung cancer presented polyuria, dysuria and diarrhea after 12 cycles of Nivolumabe (dose, schedule). Urinary sedimentoscopy revealed massive pyuria and serial urine cultures were negative (Table 1). Similarly, to the other cases described above, the symptoms improved when Nivolumab was interrupted and worsened upon re-exposure [4,13].
Our case was the first to occur after ipi/nivo combination, and symptoms were not completely controlled by oral corticoids, leading to the introduction of Infliximab. Although we did not perform a diagnostic cystoscopy, the urinary massive pyuria combined with improved outcome with the directed therapy corroborates to the hypothesis of immune-mediated cystitis. Besides, our patient continued with Nivolumab, without cystitis relapse, but maintained mild immune-related diarrhea.
The hypothesis linking the events of colitis and cystitis has been explored and were coincident in our case as well as in previous reports. This is may represent common features of immune-activation between bladder and colon as showed in mice models.
CONCLUSION
Immune-related cystitis is a rare event related to Ipilimumab/nivolumab combination that should raise suspicion when in the presence of symptoms, especially following colitis. Physicians should be aware of this rare entity, always thoroughly investigated and properly treated.
1. Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, et al. (2017) Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 28: iv119-iv142.
2. Chu E, DeVita VT (2018) Jr. Physicians' Cancer Chemotherapy Drug Manual.
3. Yervoy (ipilimumab) (2019) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb.
4. Xing P, Zhang F, Wang G, Xu Y, Li C, et al. (2019) Incidence rates of immune adverse events and their correlation with response in advanced solid tumors treated with NIVO or NIVO + IPI: A systematic review and meta-analysis. J Immunother 7: 341.
5. Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, et al. (2015) Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373: 1803-1813.
6. Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, et al. (2018) Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med 378: 1277-1290.
7. Robert C, Long GV, Brady B, Dutriaux C, Maio M, et al. (2015) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372: 320-330.
8. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, et al. (2015) Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373: 123-135.
9. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, et al. (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363: 711-723.
10. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, et al. (2012) Safety, activity and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366: 2443-2454.
11. Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, et al. (2015) Safety profile of nivolumab (NIVO) in patients (pts) with advanced melanoma (MEL): A pooled analysis. J Clin Oncol 33: 785-792.
12. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, et al. (2015) Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373: 23-34.
13. Abdel-Wahab N, Shah M, Suarez-Almazor ME (2016) Adverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reports. PLoS One 11: e0160221.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Renal Transplantation Science (ISSN:2640-0847)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- Journal of Alcoholism Clinical Research
- Journal of Immunology Research and Therapy (ISSN:2472-727X)
- Journal of Spine Diseases
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)